Navigating the Complexities of Silica in RO Systems
Silica scale is one of the most difficult scales to control, prevent, and remove from a reverse osmosis (RO) membrane system. While pretreatment techniques can provide some assistance in silica scale control, such as antiscalant, scaling events can occur due to changes in feedwater quality, loss of antiscalant injection or improper flushing during shutdown. Once on a membrane, silica scale is very difficult to remove and requires specialized cleaners. Therefore, it is important to understand the different types of silica found in water and how silica scale forms to prevent scaling events. When it comes to understanding silica scaling there are a few key points to remember:
- Silica occurs naturally in most feedwaters in the range of 1-100 mg/L
- There are two types of silica in feedwaters: dissolved (reactive) and colloidal (precipitated)
- Colloidal silica will act like a particle and foul the first membranes in the RO system
- Dissolved silica will pass through all pretreatment and can concentrate up in the RO to form silica scale
- Silica scale is highly dependent on pH and temperature
- Silica scale should be prevented, as continuous scaling can damage membranes
Testing for silica
As previously mentioned, silica can be found in natural water supplies in two forms, dissolved silica and colloidal silica. Testing for silica in feedwater is often done by testing total silica using inductive couple plasma, UV Vis spectroscopy or other ASTM standards which dissolve ALL silica species (colloidal and dissolved). Silica scale only forms when “dissolved” silica scale concentrates in the RO and becomes supersaturated. Testing for total silica can often give false measurements for dissolved silica that can limit recovery when running scaling prediction software. It is recommended that when testing for silica the species be separated through testing reactive and total silica, where the difference of the total from the reactive silica is colloidal silica or filtering a sample using a 0.45 micron filter to remove any precipitated silica from the water before the total silica test.
Colloidal Silica
Colloidal silica can be treated as a particle in water and can be removed with the same type of pretreatment. Microfiltration/ultrafiltration and media filters are the most common way to remove particles before a RO system. Additionally, cartridge filters before the RO system can also provide additional safety from particulate fouling. When membranes do foul from colloidal silica often a formulated cleaner containing dispersants can help remove the particles from the membrane surface and keep the particles suspended in solution during the clean.
Predicting Silica Scale
Unlike colloidal silica, dissolved silica cannot be removed before the RO and will pass through all pretreatment. Before you start to worry about your system scaling, there are several factors required to form silica scale. First, the silica has to be supersaturated, meaning the amount of silica concentrating in the RO system has to reach a point where the amount of silica at a specific temperature and pH exceeds a threshold where silica is no longer stable in solution. Scaling prediction software can be used to predict silica scaling but it is important to not only look at the amount of dissolved silica in the concentrate but also saturation at a specific pH and temperature. Unlike some of the projection software on the market, Avista™ Advisor™Ci Online uses silica saturation to determine antiscalant dosing. Silica saturation is a much more effective and accurate way to describe the concentration of silica and how it interacts with other ions in the water. Solely looking at the ppm of dissolved silica in the concentrate will leave out principal factors such as interaction with other ions, adjustments made due to ionic strength, ion pairing, and other chemical factors, all of which will affect the amount of soluble and insoluble silica in solution.
How silica scale forms
This part of the article will go into the chemistry of silica scale so please crack open your old chemistry text books to follow along. Silica solubility is a function of both pH and temperature. At low pH values silica is found as monosilicic acid, Si(OH)4. This molecule is in equilibrium with the silicate ion, SiO4H3-, but as the pH increases, the two molecules can bind together to form a silica dimer, Si2O7H6. The silica dimer will then continue to react with other silica dimers to polymerize silica scale as shown in image 1 below. The rate of silica polymerization and silica precipitation depends upon several factors, most of which have been only related semi-quantitatively. Increasing the pH will increase the rate of silica dimer production. Seed crystals, including already present silica depositions, will increase the nucleation rate of silica scale formation. Increasing the amount of soluble silica will increase of the available reactive silica.

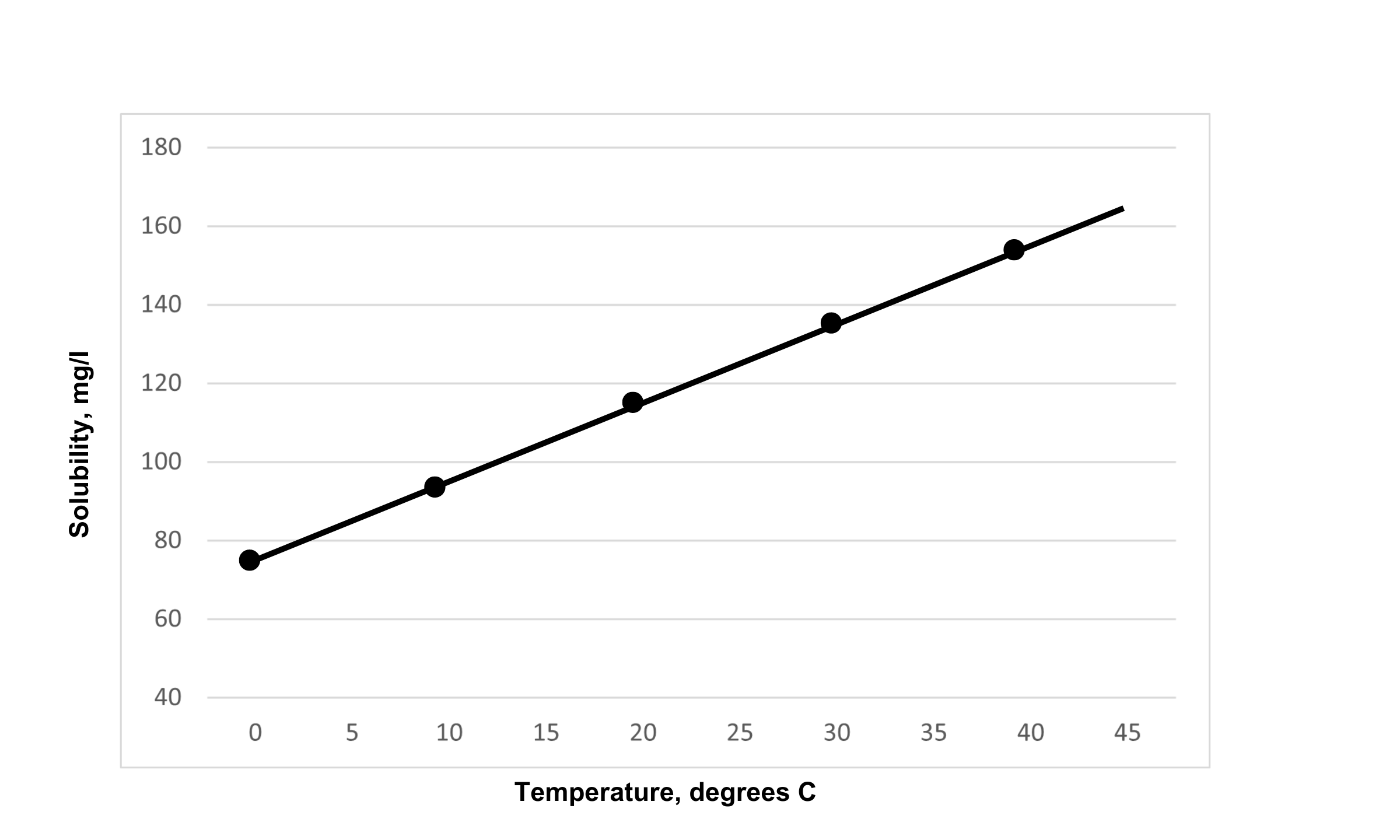
Silica solubility changes drastically with temperature. As shown in Figure 1, silica increases in solubility (x-axis) as temperature increases (y-axis). At room temperature (75F, 24C) approximately 125 ppm of soluble silica can stay in solution. However, if the temperature is increased to 86F (30C) soluble silica is closer to 135 ppm in solution.
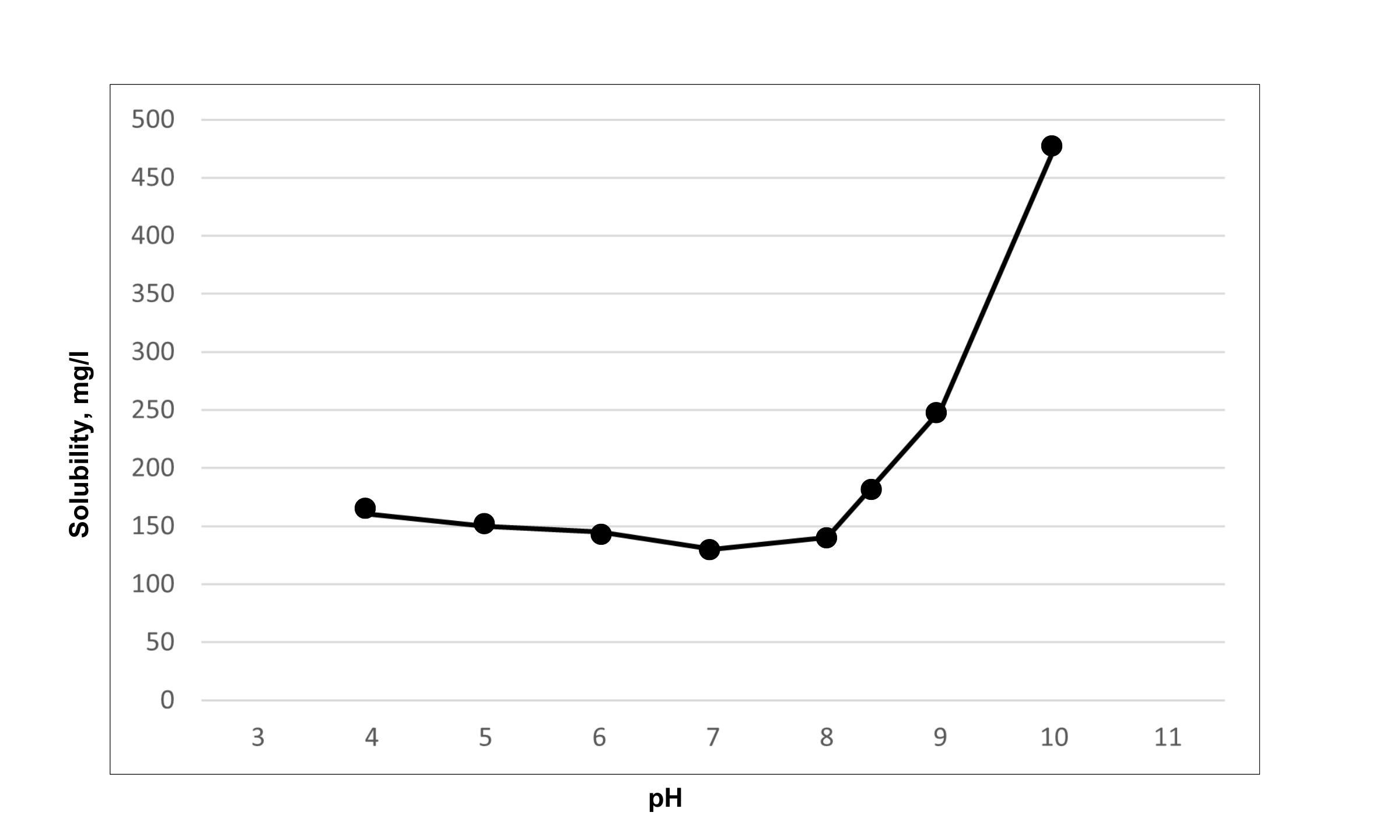
Silica also has a dramatic increase in solubility at high pH values. pH values above 8.5 will have a drastic increase in the solubility of the silica. Many high recovery systems will add additional sodium hydroxide to increase the pH of the system to increase the amount of silica that is soluble in solution. Figure 2 shows the silica solubility vs pH when temperature is held constant. In the low pH range < 5.0, although silica is not very soluble, it is in an acid form of silica (monosilicic acid), which can form silica scale but at a much lower rate.
Silica scale prevention
When membranes are scaled with silica, they will lose flow which can show in the system data as an increase in feed pressure to compensate for the loss of flow. Additionally, conductivity of the permeate will increase. Silica acts like a very thin layer of glass cutting off flow to the membrane surface. During membrane autopsy, the membrane often looks clean and weight of the element does not increase because the layer is so thin. Threshold inhibitors designed to prevent crystalline scales (such as calcium sulfate and calcium carbonate) have little inhibitory effect on silica. Polymerized silica is amorphous in nature with no defined crystal structure. To prevent silica polymerization we rely on dispersant polymers to form electrostatic and steric barriers around developing silica nuclei. This prevents their contact and growth. Often waters can contain the potential for more than one scale, so specialized silica scale antiscalants will sometimes contain both a dispersant and threshold inhibitor to prevent both silica and crystalline scales such as calcium carbonate, such as Vitec 4000.
Repeated silica scaling episodes can damage RO membrane surfaces. Silica scale is hard to remove and when on the membrane surface can cause abrasion. For this reason, it is far better to prevent silica scale than to treat its symptoms by cleaning. However, when silica scaling does occur, there are some cleaning agents that can remove it. One common cleaner is ammonium bifluoride which creates hydrofluoric acid when added to water. Hydrofluoric acid is extremely reactive and can cause burns on skin if not properly used. Additionally, hydrofluoric acid also corrodes pressure vessel surfaces and fiberglass element wrappings. Alkaline cleaners such as RoClean P112 have been developed as a safer alternative. These cleaners do not contain strong acids and are not classified hazardous and are effective in removing silica scale. Avista strongly recommends RoClean P112 cleaner over ammonium bifluoride for safety and RO component compatibility.
Silica presents a unique challenge to RO systems with its distinctive scaling mechanism and cleaning requirements. RO system operators should be aware of its specific requirements and use tools available like AdvisorCi to confirm if this is a potential scale in their RO system. If identified Avista can help recommend the right chemistry to prevent silica scale and restore membranes safely.



